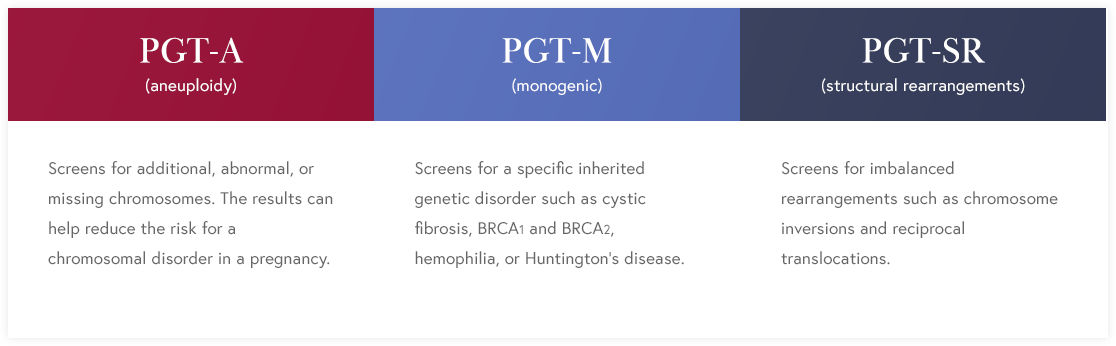
Preimplantation Genetic Testing (PGT)
What Is Preimplantation Genetic Testing (PGT)?
Preimplantation genetic testing is a reproductive technology used with an in vitro fertilization (IVF) cycle to increase the potential for a successful pregnancy and delivery. PGT is a genetic test on cells removed from appropriately developing embryos to help select the best embryo(s) for transfer to the uterus. This can lead to an increased implantation rate, decreased miscarriage rate, and improved success rate for delivering a healthy baby.
Why Should I Use Genetics & IVF Institute for PGT?
Genetics & IVF Institute has long been a leader in PGT for genetics disease prevention. PGT is performed at our onsite laboratory, Fairfax Diagnostics. Since testing takes place at our facility you need not worry about your embryo biopsies being sent to an off-site lab. You’ll also have the benefit of comprehensive and coordinated care between the embryology laboratory, PGT laboratory, and clinical staff, who are in a single location.